Added
Want More Information?
You’ve Come To The Right Place
Sheathing Technologies strongly embraces an ongoing commitment to creating the best products in the industry to offer top to bottom protection. This includes the world’s only FDA-Cleared Viral Barrier Ultrasound probe covers, which have been extensively tested against all bacteria and testable viruses down to 20nm for maximum safety for your infectious control needs and unequaled overall quality.
Read and review
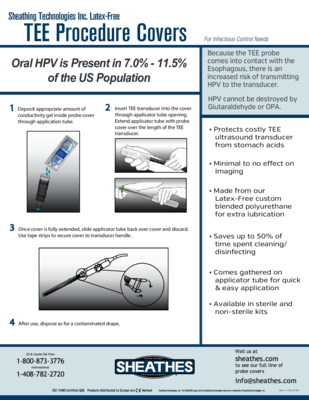
Download our marketing brochures and instructional pamphlets, review our publication briefs, and look at our new product media center.
Brochures / Informational Pamphlets
Publication Briefs
Copyright © Sheathing Technologies, Inc. 2021. | All Rights Reserved. | Patents | Privacy policy